- Facility
Biophysics & Structural Biology (B2S)
- Microcalorimetry, Circular Dichroïsm, Surface Plasmon Resonance, NMR, Chromatography, Light Scattering, Fast kinetics, Cryomicroscopy
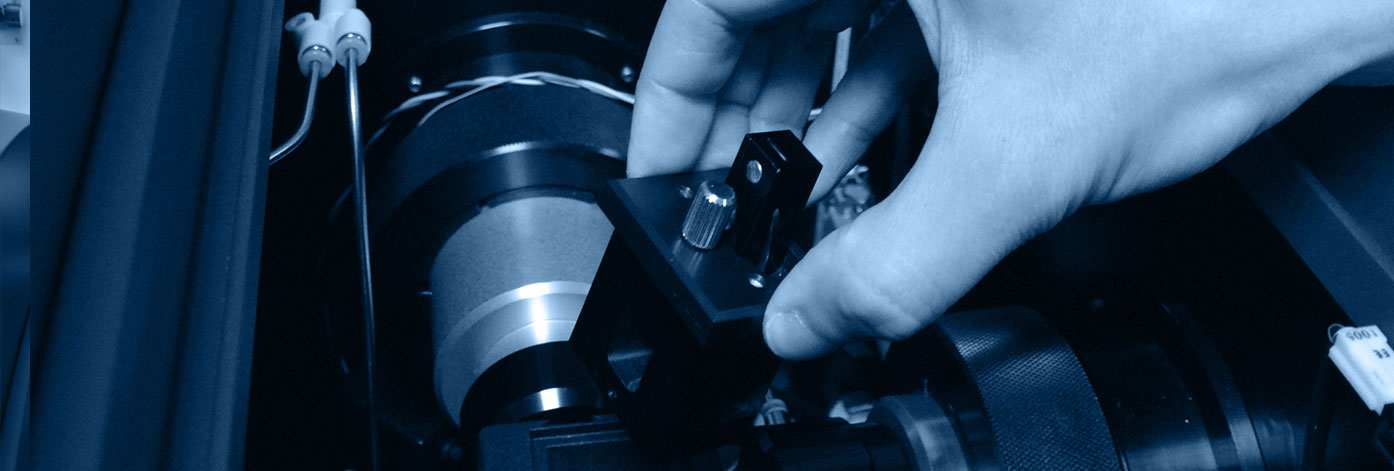
The Biophysics and Structural Biology (B2S) platform offers a combination of equipment for in vitro physicochemical characterization of proteins and interactions. It provides access to several biophysical (circular dichroism, microcalorimetry, surface plasmon resonance, fluorescence) and structural biology (NMR, X-ray) devices, and is based on the skills of the IMoPA research teams. It belongs to the ARBRE association (Association of Resources for Biophysical Research in Europe) created in 2015. It allows the development of multidisciplinary projects to characterize 1) the interactions involved between biological macromolecules and, 2) the 3D structure biological macromolecules.
The B2S staff has a real technical and methodological expertise to address different issues: The stability / characterization of proteins and complexes in solution, the three-dimensional structure of proteins and complexes, fast kinetics, the characterization of protein-protein and protein-ligand interactions as well as the isolation and characterization of a broad spectrum of products by liquid chromatography.
The platform operates in two modes: 1) the service mode (turnkey services along with, or not, collaborations) and 2) the provision of devices for expert users after initial training.
Any new collaborator must contact the person in charge of the Core Facility to define the best strategy to adopt for the project and complete the service request form. Any user will accept the terms and conditions for service use by signing the agreement form.
We are always available for users during the use of the devices and for any advice and assistance.
Each user must retrieve its data at the end of the analyses. In the usual case, the raw acquisition data are kept for 1 year on computer stations. The platform does not guarantee the recovery of data in case of malfunction. The user is responsible for the final data storage.
Lec J.C, Boutserin S, Mazon H, Mulliert G, Boschi-Muller S, Talfournier F. Unraveling the Mechanism of Cysteine Persulfide Formation Catalyzed by 3-Mercaptopyruvate Sulfurtransferases. ACS Catal. 2018 ; 8(3):2049–2059.
 10.1021/acscatal.7b02432 ,
10.1021/acscatal.7b02432 ,  HAL-01715178
HAL-01715178 Carrasco K, Boufenzer A, Jolly L, Le Cordier H, Wang G, Heck AJ, Cerwenka A, Vinolo E, Nazabal A, Kriznik A, Launay P, Gibot S, Derive M. TREM-1 multimerization is essential for its activation on monocytes and neutrophils. Cell Mol Immunol. 2018 Mar 22.
 10.1038/s41423-018-0003-5 ,
10.1038/s41423-018-0003-5 ,  29568119 ,
29568119 ,  HAL-01945617
HAL-01945617 Bragantini B, Rouillon C, Charpentier B, Manival X, Quinternet M. NMR assignment and solution structure of the external DII domain of the yeast Rvb2 protein. Biomol NMR Assign. 2018 Oct ; 12(2):243-247.
 10.1007/s12104-018-9816-5 ,
10.1007/s12104-018-9816-5 ,  29569106 ,
29569106 ,  HAL-01882005
HAL-01882005 Maurizy C, Quinternet M, Abel Y, Verheggen C, Santo PE, Bourguet M, Paiva A, Bragantini B, Chagot ME, Robert MC, Abeza C, Fabre P, Fort P, Vandermoere F,Sousa P Rain JC, Charpentier B, Cianférani S, Bandeiras TM, Pradet-Balade B, Manival X, Bertrand E. The RPAP3-Cterminal domain identifies R2TP-like quaternary chaperones. Nat Commun. 2018 May 29 ; 9(1):2093.
 10.1038/s41467-018-04431-1 ,
10.1038/s41467-018-04431-1 ,  29844425 ,
29844425 ,  HAL-01872981
HAL-01872981 Henri J, Chagot ME, Bourguet M, Abel Y, Terral G, Maurizy C, Aigueperse C, Georgescauld F, Vandermoere F, Saint-Fort R, Behm-Ansmant I, Charpentier B, Pradet-Balade B, Verheggen C, Bertrand E, Meyer P, Cianférani S, Manival X, Quinternet M. Deep Structural Analysis of RPAP3 and PIH1D1, Two Components of the HSP90 Co-chaperone R2TP Complex. Structure. 2018 Sep 4 ; 26(9):1196-1209.
 10.1016/j.str.2018.06.002 ,
10.1016/j.str.2018.06.002 ,  30033218 ,
30033218 ,  HAL-01919243
HAL-01919243 Kriznik A, Yéléhé-Okouma M, Lec JC, Groshenry G, Le Cordier H, Charron C, Quinternet M, Mazon H, Talfournier F, Boschi-Muller S, Jouzeau JY, Reboul P. CRDSAT Generated by pCARGHO: A New Efficient Lectin-Based Affinity Tag Method for Safe, Simple, and Low-Cost Protein Purification. Biotechnol J. 2018 Oct 8:e1800214.
 10.1002/biot.201800214 ,
10.1002/biot.201800214 ,  30298550 ,
30298550 ,  HAL-01892441
HAL-01892441 Yakavets I, Lassalle HP, Yankovsky I, Ingrosso F, Monari A, Bezdetnaya L, Zorin V. Evaluation of temoporfin affinity to beta-cyclodextrins assuming self-aggregation. Journal of Photochemistry and Photobiology A: Chemistry. 2018 Dec 1.
 10.1016/j.jphotochem.2018.07.046
10.1016/j.jphotochem.2018.07.046 Dorival J, Risser F, Jacob C, Collin S, Dräger G, Paris C, Chagot B, Kirschning A, Gruez A, Weissman KJ. Insights into a dual function amide oxidase/macrocyclase from lankacidin biosynthesis. Nat Commun. 2018 Sep 28 ; 9(1):3998.
 10.1038/s41467-018-06323-w ,
10.1038/s41467-018-06323-w ,  30266997 ,
30266997 ,  HAL-01980537
HAL-01980537 Bersweiler A, D'Autréaux B, Mazon H, Kriznik A, Belli G, Delaunay-Moisan A, Toledano MB, Rahuel-Clermont S. A scaffold protein that chaperones a cysteine-sulfenic acid in H(2)O(2) signaling. Nat Chem Biol. 2017 Aug ; 13(8):909-915.
 10.1038/nchembio.2412 ,
10.1038/nchembio.2412 ,  28628095 ,
28628095 ,  HAL-01652643
HAL-01652643 Roy O, Dumonteil G, Faure S, Jouffret L, Kriznik A, Taillefumier C. Homogeneous and Robust Polyproline Type I Helices from Peptoids with Nonaromatic α-Chiral Side Chains. J Am Chem Soc. 2017 Sep 27 ; 139(38):13533-13540.
 10.1021/jacs.7b07475 ,
10.1021/jacs.7b07475 ,  28837348 ,
28837348 ,  HAL-01656039
HAL-01656039