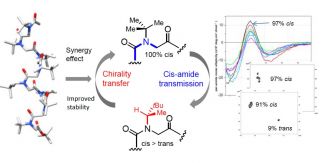
Thanks to a circular dichroism analysis carried out on the Biophysics and Structural Biology (B2S) Core Facility, the structural stability of peptoids in the all-cis Polyproline I form has been demonstrated and new conditions for organic synthesis have been developed.
The synthesis of biomimetic helical secondary structures is highly sought after for the construction of innovative nanomaterials with possible medical applications such as the development of protein-protein interaction modulators.
Peptoids, a family of peptidomimetic oligomers, have a propensity for helix structure. However, cis-trans isomerization of the tertiary skeleton may hinder the adoption of stable secondary structures by the peptoid, including the helical all-cis Polyproline I conformation.
By coupling circular dichroism and NMR analytical methods, we show here that NtBu monomers, strategically positioned in the chiral sequences, can enhance and stabilize the degree of helicity of peptoids even with a reduced content of chiral side chains.
Publication associée : Rzeigui M, Traikia M, Jouffret L, Kriznik A, Khiari J, Roy O, Taillefumier C. Strengthening Peptoid Helicity through Sequence Site-Specific Positioning of Amide Cis-Inducing NtBu monomers. J Org Chem. 2019 Dec 24.
DOI : 10.1021/acs.joc.9b02916
PMID : 31873018
HAL : HAL-02430577