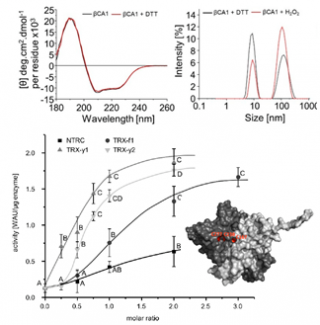
Carbonic anhydrase is subject to redox regulation by thioredoxins, indeed inactive in its oxidized form and active in its reduced form, it is finely regulated to allow the accumulation of CO2 from carbonate HCO3- within the chloroplasts of C3-plants, and thus optimize the process of photosynthesis.
Thanks to the resources of the B2S Core Facility (circular dichroism and dynamic light scattering), researchers from the University of Bielefeld (Germany), in collaboration with the UMS IBSLor, have highlighted the oligomeric state of A.thaliana carbonic B-anhydrase: a homodimer with a hydrodynamic diameter of between 7 and 8 nm, as well as its sensitivity to oxidation, only slightly disturbing its structuring (increased presence of aggregated forms). T
hioredoxin-type reducing proteins and especially TRX-y1, TRX-y2, are capable of restoring the activity of oxidized β-CA while avoiding disturbing its quaternary structure as demonstrated by CD and size exclusion chromatography.
For more information on this research: Dreyer A, Schackmann A, Kriznik A, Chibani K, Wesemann C, Vogelsang L, Beyer A, Dietz KJ. Thiol Redox Regulation of Plant β-Carbonic Anhydrase. Biomolecules. 2020 Jul 30.
DOI: 10.3390/biom10081125
PUBMED: 32751472
HAL: HAL-02917419