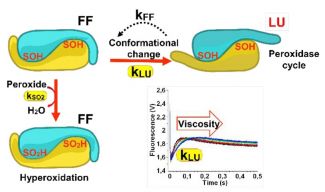
UMS2008/US40 IBSLor and UMR7365 IMoPA rapid kinetics expertise in the circular dichroism and fluorescence modes led to establish the kinetics of a determinant conformational change in the molecular mechanism and regulation of the antioxidant enzyme Peroxiredoxin.
Redox biology is an increasingly recognized research field that addresses the regulation of major processes including aging and associated diseases by reactive species, as the oxidant peroxides. The Peroxiredoxins are major cellular antioxidants that scavenge peroxides. They are moonlighting enzymes regulated by Cysteine thiol oxidation, including into the sulfinic acid state (sulfinylation). Recently sulfinylation was recognized as a widespread protein modification that could be involved in the function of many proteins in human cells. Peroxiredoxins offer a major model of protein-thiol oxidative modification.
Their differential sensitivity to sulfinylation depends on a molecular motion switch occurring within the protein structure, in the course of the catalytic cycle. By using molecular enzymology and rapid kinetics approaches, we show that sulfinylation sensitivity is strictly dependent on the competition occurring within the protein between the molecular motion and the chemical sulfinylation processes. This allows to predict Peroxiredoxins relative sulfinylation sensitivity in vitro and in vivo. Our work sets the bases for understanding the mechanism of protein Cysteine sulfinylation in general, a critical issue with regards to the importance of redox responses in numerous physiopathological conditions.
Publication: Kriznik A, Libiad M, Le Cordier H, Boukhenouna S, Toledano MB, Rahuel-Clermont S. Dynamics of a key conformational transition in the mechanism of peroxiredoxin sulfinylation. ACS Catalysis. 2020.
DOI:10.1021/acscatal.9b04471
HAL: HAL-02473398