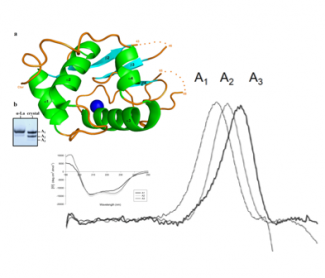
Thanks to the resources of the B2S Core Facility (X-ray diffraction, circular dichroism and micro calorimetry), a collaborative research from the University of Tizi-Uzou, Algeria (S. Si Ahmed Zennia & A. Mati) and the University of Lorraine (URAFPA ENSAIA, J-M. Girardet & C. Cakir-Kiefer and IMoPA, C. Charron & A. Kriznik) has shown that deamidation reinforced the thermostability and resistance to proteolysis of the a-lactalbumin, and might induce formation of a short extra α-helix.
This work, published in Food Chemistry, illustrates how the essential proteins of these desert animals have evolved in order to overcome the high temperatures encountered in these latitudes.
Publication: S.Si Ahmed Zennia, A.Mati, C.Charron, C.Cakir-Kiefer, A.Kriznik, J-M.Girardet. Effect of nonenzymatic deamidation on the structure stability of Camelus dromedarius alpha-lactalbumin. Food Chemistry. 2019 April 8